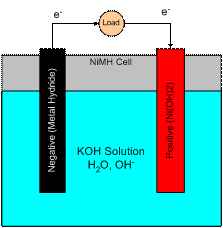
The type of battery used in our robot and for our project is a Nickel Metal Hydride battery. It is important to understand how the battery works if we attempt to charge it and make our project based around it. The following are the half reactions for each anode and the overall reaction explained. An image of a NiMH battery is shown below.
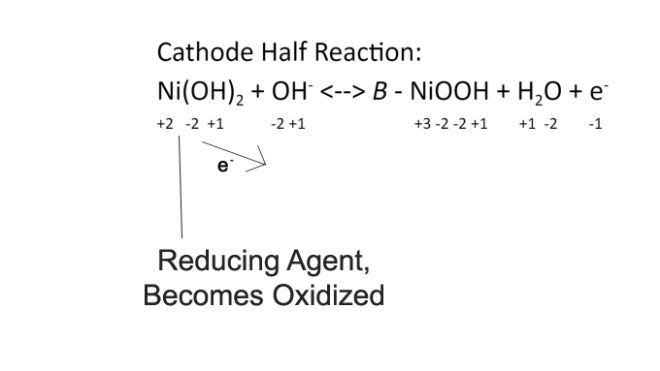
Cathode Half Reaction: Ni(OH)2 + OH– <–> B – NiOOH + H2O + e–
This reaction shows the reaction in both directions — left to right charges the battery, right to left discharges it. In the cathode, the second H in Ni(OH2) moves to the OH– to create a stable H2O molecule. In this process, an electron would be left over because of the negative charge on the OH molecule. This electron is released out of the cathode and continues through the circuit. The B – NiOOH section of the right of the equation is a semiconductor with B (beta) signifying the amount of energy reduced in the resistor. The H2O molecule is a byproduct.
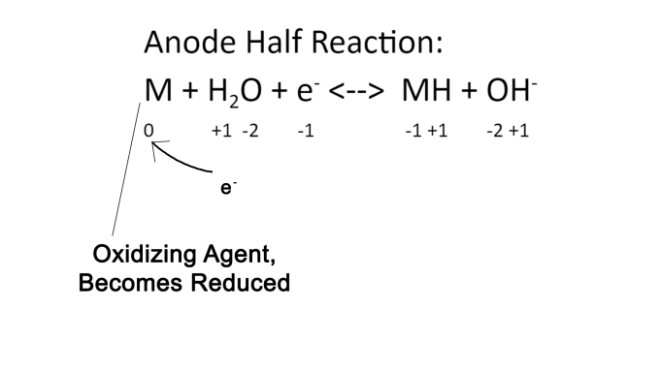
Anode Half Reaction: M + H2O + e– <–> MH + OH–
In the Anode reaction, an electron is received that is released from the cathode reaction. This is the e– shown on the left side of the equation. The M in this equation depends on the type of the battery. In Nickel Metal Hydride batteries, there are a variety of configurations that include different types of metal that can replace the M. These are called AXBX forms. The four types are AB5, AB2, AB, and A2B. In each of these forms, the A can be a couple different atoms and the B can be different atoms. In the type of battery used in my project, the form is AB5. The A represents Lanthanum and the B represents Cobalt. In this particular form, the A is always a rare earth metal such as Lanthanum, Cerium, and Titanium. The B is always a fairly common conductive metal such as Cobalt, Nickel, Aluminum, or Manganese. The full equation with the specific metals substituted in for this battery would be LaCo5 + H2O + e <–> LaCo5H + OH–. This equation shows the metal of the anode as M receiving H2O and the electron released by the Cathode, and changes them to a form much more similar to what the Cathode begins with. The anode effectively resets the molecules back to what is used in the Cathode equation.
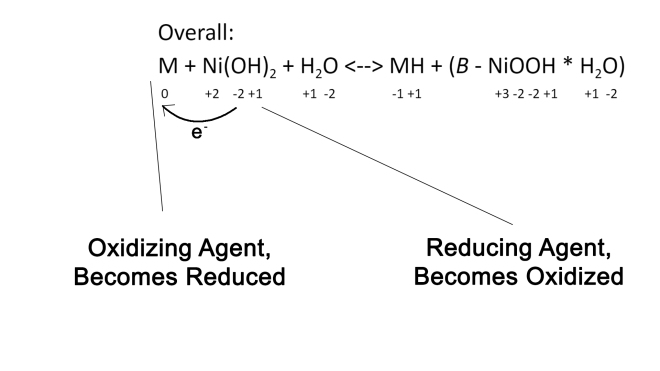
Overall: M + Ni(OH)2 + H2O <–> MH + (B – NiOOH * H2O)
This is the overall reaction of the battery. On either side of the reaction there is an M and an Ni, which are the solid components of the cathode and anode that cannot be changed without the battery physically falling apart. The Oxygen and Hydrogen atoms on either side being moved around as they are in the individual Anode and Cathode reactions. We can see the semiconductor in the cathode reaction showed in the right side of this reaction. This semiconductor regulates the current that’s coming out of the battery.
Equations w/ Redox Details (Recharge is left to right):